Deep screening of sybodies directed against SARS-CoV-2 using a high-throughput nano-immunoassay
A large number of synthetic antibody variants will be fed into an in vitro selection and enrichment process, resulting in a pool of potentially high-value neutralising antibody candidates. These variants will then be isolated, synthesised and screened for their properties in a high-throughput nano-immunoassay to identify the most promising candidates.
Background and scientific basis
Neutralising antibodies, scFVs, nanobodies and sybodies emerged as promising prophylactics/therapeutics for SARS-CoV-2 infections. By coupling in vitro display methodologies to a high-throughput microfluidic approach to characterising the binding between antibody and S-protein, a large number of candidate molecules can be characterised in vitro and selected for further development stages.
Problem and approach to solution
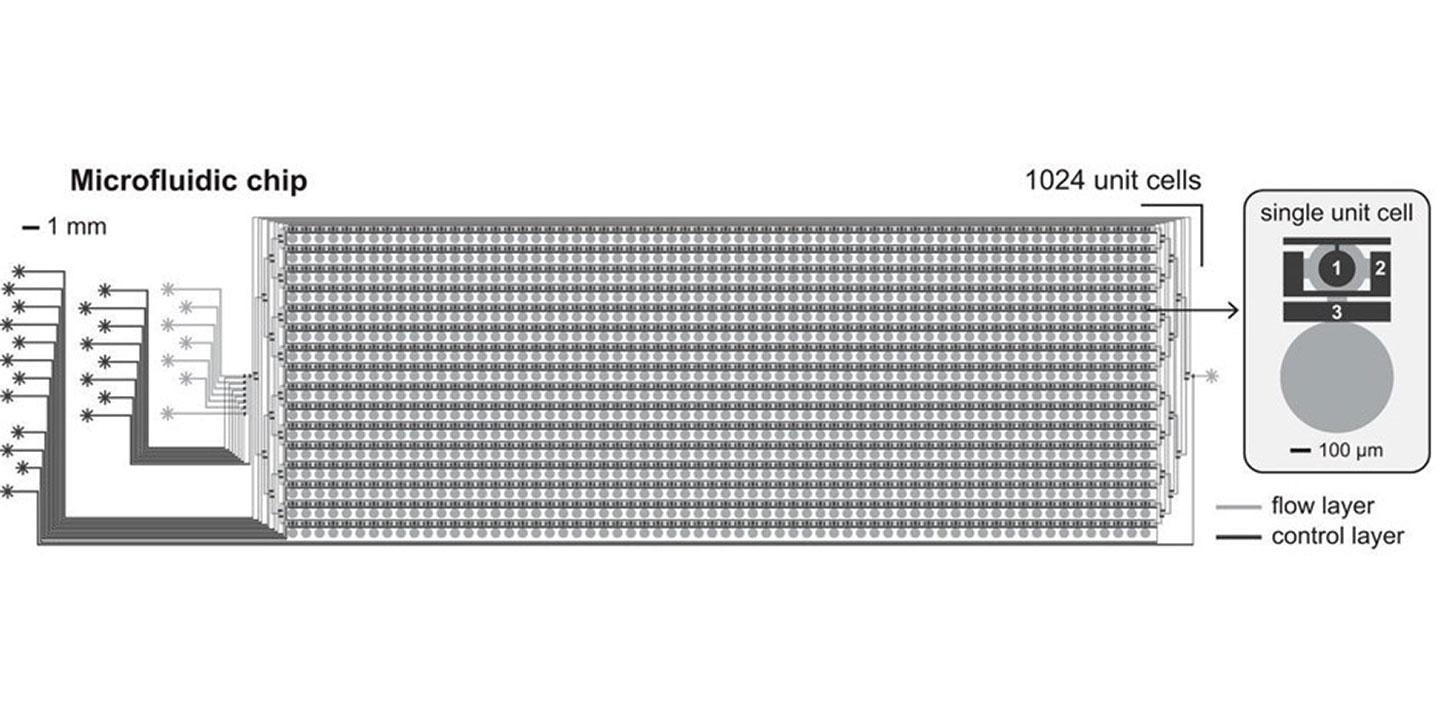
Therapeutic antibody discovery involves long timelines and is cumbersome and expensive. The Seeger lab at the University of Zurich has developed a new generation of neutralising sybodies (synthetic single domain antibodies) with high therapeutic potential, which have been targeted at the S-protein of SARS-CoV-2. The Maerkl lab at EPF Lausanne has developed a microfluidic nano-immunoassay (NIA) capable of characterising the binding of SARS-CoV-2 specific antibodies to S-protein in high-throughput. Coupling these methodologies with cell-free protein synthesis will allow for a large number of candidate molecules to be rapidly characterised in detail to identify leading candidates with improved binding characteristics.
Expected output and contribution to tackling the pandemic
The project will aid the discovery of a set of sybodies with therapeutic promise in terms of their binding properties. Furthermore, the development of this experimental pipeline will ultimately enable therapeutically promising antibodies against SARS-CoV-2 and SARS-CoV-2 variants to be discovered faster. This study will also demonstrate the potential of using the “mechanically induced trapping of molecular interactions” (MITOMI) platform in combination with cell-free protein synthesis as a high throughput screening and characterisation approach in order to facilitate the rapid development of affinity reagents in general.
NRP 78 research projects
