New technology for decentralised and low-cost mass testing
The research group developed a large-scale serological profiling of SARS-CoV-2 and related human CoVs with high-throughput microfluidic nano-immunoassays.
Background
To perform sero-surveillance requires the use of immunoassays for the detection of virus specific antibodies in human blood. Existing assays require the use of serum, which must be obtained by a venous blood draw by a trained medical professional, and this procedure generally needs to be performed at a point of care such as a hospital or clinic. In this project, we addressed these aspects among others by developing a novel serological assay as well as by implementing it immediately in a joint epidemiological-virological study.
Aim
We aimed to develop new technologies to perform highly accurate, quantitative immunoassays in high-throughput and at low-cost. We wanted to eliminate the need for serum and concomitant venous blood draws by developing approaches to collect ultra-low volume capillary blood samples obtainable by simple fingerpick. These approaches were to be fully characterized and validated and these technologies were to be deployed in sero-surveillance programmes in the Geneva and Lausanne area.
Results
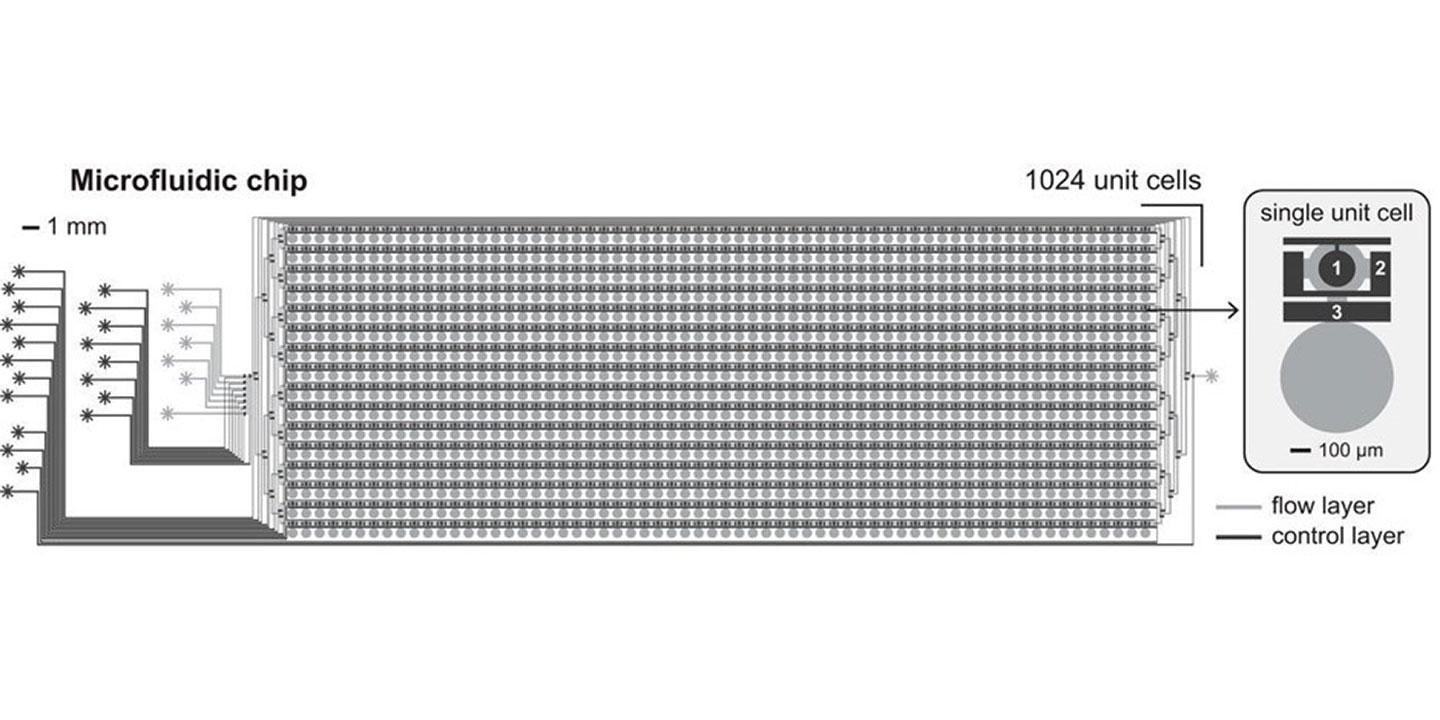
We set out to develop next generation technology to eliminate the major bottlenecks of existing molecular diagnostic methods. To do so, we developed a high-throughput, low-cost microfluidic system capable of analyzing over 1’000 samples in parallel while reducing the cost of the assay to 0.06% for reagents alone as compared to standard ELISA tests. Our new method outperforms existing technologies in terms of sensitivity and specificity, which are amongst the most important parameters determining the usefulness of a molecular diagnostics assay. Furthermore, we developed up-stream sample collection and processing steps that permit untrained individuals to perform a simple fingerpick, collect a minute volume of capillary blood, and send this sample by regular postal mail to be analyzed on our next-generation molecular diagnostics platform. This enables low-cost, simple, and convenient de-centralized sample collection, coupled with highly precise centralized analysis.
We also analyzed the role of SARS-CoV-2-specific locally produced s-IgA antibodies in the nasal mucosa and compared them to functional neutralizing antibodies. We demonstrated that previous infection elicits significantly higher SARS-CoV-2 secretory component IgA (s-IgA) antibody responses, while vaccination leads to the s-IgA responses only in a minority of individuals. In addition, boosting by a third vaccine dose does not improve these responses. We showed that protection by neutralization against different SARS-CoV-2 strains was higher in previously infected individuals compared to those vaccinated only. Interestingly, neutralization of Omicron BA.5 strain was comparable in individuals with previously confirmed BA.1 or Delta SARS-CoV-2 infections. Furthermore, there is strong evidence that s-IgA substantially contributes to virus neutralization in the nasal mucosa.
Concerning viral shedding, we demonstrated that vaccination with 2 doses reduced infectious viral loads in Delta-infected individuals, whereas for Omicron BA.1 breakthrough cases, reduced infectious viral load was observed only in boosted individuals, but not in individuals vaccinated with 2 doses compared to unvaccinated individuals.
Specific contribution to tackle the current pandemic
We were able to rapidly develop and validate new technologies enabling large-scale sero- surveillance and deployed these in Geneva and Lausanne to support sero-surveillance programmes in this region. The use of this assay could contribute to findings on seroconversion in the general population as well as in children and enable child-friendly outbreak-investigations. Our findings on infectious viral loads indicate that vaccines may lower transmission risk for Delta and Omicron BA.1 variants of concern and, therefore, have a public health benefit beyond individual protection.
Original title
Large-scale serological profiling of SARS-CoV-2 and related human CoVs with high-throughput microfluidic nano-immunoassays
